- GENERAL INFORMATION ABOUT THE JOURNAL
- Focus and Scope
- Editorial Board
- Data
- Official Journal of UMEMPS, the Portuguese Neonatal Society, the Hellenic Neonatal Society, the Hellenic Society of Perinatal Medicine, IRPS, SIN INF, S.I.P.Ped, SIPO and SIMPE
- Metrics
- Publication Ethics
- Disclaimer
- Open Access Policy
- Academic re-use
- Commercial re-use
- Advertising Policy
- INFORMATION FOR AUTHORS
- Submissions
- Publishing in JPNIM is free of charge
- The steps toward publishing in JPNIM
- Screening for plagiarism
- Peer Review Process
- Scientific copyediting
- Copyright and publishing rights
- Academic re-use
- Self-archiving
- Archiving
- Indexing
- Corrections, retractions, and editorial expressions of concern
- Declarations to be made by the authors
GENERAL INFORMATION ABOUT THE JOURNAL
Focus and Scope
The Journal of Pediatric and Neonatal Individualized Medicine (JPNIM) is a peer-reviewed interdisciplinary journal which provides a forum on new perspectives in pediatric and neonatal medicine. The aim is to discuss and to bring readers up to date on the latest in research and clinical pediatrics and neonatology. Special emphasis is on developmental origin of health and disease or perinatal programming and on the so-called ‘-omic’ sciences. Systems medicine blazes a revolutionary trail from reductionist to holistic medicine, from descriptive medicine to predictive medicine, from an epidemiological perspective to a personalized approach. The journal will be relevance to clinicians and researchers concerned with personalized care for the newborn and child. Also medical humanities will be considered in a tailored way.
Article submission (original research, review papers, invited editorials and clinical cases) will be considered in the following fields: fetal medicine, perinatology, neonatology, pediatrics, developmental programming, psychology and medical humanities.
Editorial Board
Please find the Editorial Board on the dedicated webpage.
Data
Journal Name: Journal of Pediatric and Neonatal Individualized Medicine
Journal Abbreviation: J Pediatr Neonat Individual Med
Journal Initials: JPNIM
Serial Publication Number (ISSN online): 2281-0692
Publishing Frequency: six-monthly (2 issues/year)
First Issue's Date: October 2012
Publishing Mode: peer-reviewed, online, no-fee and Open Access
Publisher: Hygeia Press di Corridori Marinella, via Montecatini 53, 09045 Quartu Sant'Elena (CA), Italy - www.hygeiapress.com
Contact: JPNIM Staff - journal@jpnim.com
Managing Editor: Eleonora Fanos
Copyright: © Hygeia Press
Authorization under Italian law: Aut. Trib. Cagliari n. 16/12 del 07/06/2012, iscrizione ROC n. 22729 del 10/07/2012
Official Journal of UMEMPS, the Portuguese Neonatal Society, the Hellenic Neonatal Society, the Hellenic Society of Perinatal Medicine, IRPS, SIN INF, S.I.P.Ped., SIPO and SIMPE
Official Journal UMEMPS (Union of Middle-Eastern and Mediterranean Pediatric Societies)
Official Journal of the Portuguese Neonatal Society (Sociedade Portuguesa de Neonatologia, affiliated to the Portuguese Paediatric Society)
Official Journal of the Hellenic Neonatal Society (Ελληνική Νεογνολογική Εταιρεία)
Official Journal of the Hellenic Society of Perinatal Medicine (Ελληνική Εταιρεία Περιγεννητικής Ιατρικής)
Official Journal of the Italian-Romanian Pediatric Society (IRPS)
Official Journal SIN INF (Italian Neonatal Nursing Society, Società Italiana di Neonatologia Infermieristica)
Official Journal S.I.P.Ped. (Italian Society of Pediatric Psychology, Società Italiana di Psicologia Pediatrica) • S.I.P.Ped. manages the "Pediatric Psychology and related issues" Section (Sezione "Psicologia Pediatrica e dintorni")
Official Journal SIPO (Italian Society of Hospital Pediatrics, Società Italiana Pediatria Ospedaliera)
Official Journal SIMPE (Italian Society of Pediatric Physicians, Società Italiana Medici Pediatri) • SIMPE manages the “360° PEDIATRICS – PEDIATRIA 360°” Section
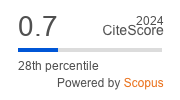
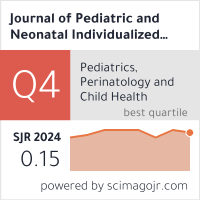
Metrics
Metrics: CiteScore 2024 is 0.7 according to Scopus; SJR 2024 is 0.15 according to SJR - Scimago Journal & Country Rank; H5-index (2019-2023) is 9 according to Google Scholar.
Publication Ethics
Our ethic statements are inspired by COPE’s Best Practice Guidelines for Journal Editors.
In particular:
- the Editor-in-Chief ensures that all published papers of research have been reviewed by qualified reviewers;
- reviewers and editors are expected to treat manuscripts fairly and confidentially and to declare any competing interests;
- the journal has published detailed publishing procedures and author guidelines;
- JPNIM staff routinely performes random controls to detect plagiarised text;
- the journal has published a policy on protecting individual data (e.g., identifiable patient details);
- authors are expected to be aware of and adhere to best practices in publication ethics and must also comply with JPNIM’s policies on research ethics.
Disclaimer
Disclaimer. Care has been taken to confirm the accuracy of the information presented and to describe generally accepted practices. However, the authors, editors, and publisher are not responsible for errors or omissions or for any consequences from application of the information in the journal and make no warranty, expressed or implied, with respect to the currency, completeness, or accuracy of the contents of this publication.
Drug dosage. The authors, editors and publisher have exerted every effort to be certain that drug selection and dosage set forth in this issue are in accordance with current recommendations and practice at the time of publication. However, in view of ongoing research, changes in government regulations, and the constant flow of information relating to drug therapy and drug reactions, the reader is urged to check the package insert for each drug for any change in indications and dosage and for added warnings and precautions. This is particularly important when the recommended agent is a new or infrequently employed drug.
All rights reserved. All materials are protected by Italian law and by international conventions. No part of this work may be translated into other languages, reproduced or utilized in any form or by any means without the prior written permission of the publisher.
Open Access Policy
This journal provides immediate open access to its content on the principle that making research freely available to the public supports a greater global exchange of knowledge.
In particular, all content is freely available without charge to the user or his/her institution. Users are allowed to read, download, copy, distribute/print a copy for personal and non-commercial use, search, or link to the full texts of the articles in this journal without asking prior permission from the publisher or the author. This is inspired by the BOAI (Budapest Open Access Initiative) definition of open access.
Academic re-use
Please note that authors have the opportunity to reuse figures, tables and selected text up to 250 words from their articles as finally published, providing that full and accurate credit shall be given to publication in JPNIM and that modifications are noted (otherwise no changes may be made). Translations are subject to the same rules. For all other academic re-use purposes, please send a request for permission to re-use material to journal@jpnim.com; please state “Academic re-use” in the subject line of your email.
Commercial re-use
Whole article re-use or parts of them (in print or electronic copies) can be useful promotional tools at industry-specific exhibitions, workshops, conferences and meetings; they can also be used for commercial and marketing purposes by corporate sales representatives. Commercial re-use includes all uses of JPNIM articles for commercial, promotional, marketing or monetary purposes. Such re-use requires further explicit permission and will be subject to a fee. Commercial re-use includes: all the proactive supplies of multiple print or electronic copies of articles or parts of them taken from JPNIM to third parties on a systematic basis; re-use by a non-author/third party/other publisher of articles or parts of them in another print or electronic publication; re-use by authors of their articles or parts of them, as not otherwise specified in the “Academic re-use” section. Those wishing to re-use parts or all of an article or articles (in print or electronic copies) for commercial purposes are required to pay our commercial rates. There can be special agreements according to which the publication of conference/workshop proceedings and dedicated special collections of articles is paid by Scientific Societies/Congress Organizers to JPNIM. Income received helps us to keep our journal freely accessible to readers and not to charge authors any fee for article processing. To request permission and obtain a quotation please contact journal@jpnim.com; please state “Commercial re-use” in the subject line of your email.
Advertising Policy
All advertisements are subject to the approval of the Publisher, who reserves the right to reject or delete any advertisement at any time. All advertisements are accepted and published by the Publisher upon the assurance of the agency and/or advertiser that they are both authorized to publish the entire content and subject matter of the advertisement. In consideration of the publication of an advertisement, the advertiser and/or agency, jointly and separately, agree to indemnify and hold harmless the Publisher and its employees from expenses (including attorney's fees) and losses arising from the publication of the content of the advertisement, including, without limitation, claims or lawsuits for defamation, invasion of privacy, copyright infringement, or plagiarism. All advertisements must clearly and visibly identify the advertiser by trademark. Any reference to the Publisher or its products or services in advertising, promotional materials or merchandising by the advertiser or agency is subject to the Publisher's written approval for such use. Publisher is not liable for incidental or consequential damages resulting from errors in the display or printing of an advertisement. The Publisher may change the terms set forth herein at any time, provided that such change shall not apply to ads whose closing date precedes the announcement of the change. In the event of non-payment within the agreed terms, the Publisher reserves the right to hold the advertiser and/or its advertising agency jointly and severally liable for overdue amounts owed to the Publisher. Proprietary names of pharmaceutical products must be accompanied by the chemical, generic or official name; the quantity of all active ingredients must be indicated along with the recommended dosage. Advertiser represents and warrants that all advertisements and products advertised, particularly pharmaceutical products, comply with all applicable laws, rules and regulations of the country in which the products are marketed. Any use of the JPNIM trademark or copyrighted material for links to and from the Publisher's website must be approved in advance by the Publisher. Any unauthorized linking is prohibited. Publisher does not endorse or support any product or organization linked to its website, nor is Publisher responsible for the content of any website promoted in an ad. The use by the advertiser or its agency of technology designed to collect personally identifiable information is prohibited.
INFORMATION FOR AUTHORS
Submissions
There are different types of submissions:- regular submissions (to be done via JPNIM website),
- submissions to S.I.P.Ped. Section (to be done by email),
- submissions to SIMPE Section (to be done by email),
- submissions of conference/workshop proceedings (to be done as specified by Scientific Societies/Congress Organizers).
Publishing in JPNIM is free of charge
Regular submissions to JPNIM are free of charge, no payments are required from the Authors: JPNIM doesn’t have article submission charges nor article processing charges (APCs).
The steps toward publishing in JPNIM
For regular submissions, the steps toward publishing in JPNIM are the following:
- all authors from non-English language countries should get their initial manuscript copyedited by scientific copyediting services at author's own expense. The authors have to submit the initial manuscript along with a certificate of editing. All copyediting services are fully paid for and arranged by the authors. NB. Any submission without a certificate of editing will be automatically rejected (more information here);
- you can upload your manuscript files online (please note that, to submit articles to JPNIM's website, authors must first be registered as both readers and authors);
- the Editor-in-Chief will make a first evaluation of the article;
- if it is considered interesting, the article will be sent to 2/3 reviewers, who will evaluate it accordingly with a “single-blind peer review” procedure;
- the article will be returned to you with the reviewers’ notes and possibly with changes to be made;
- once you have made the corrections, the article will be forwarded to the reviewers, who will again write their opinions, requiring further corrections or giving their consent to publication;
- a layout version as a PDF file will be prepared, and the proofs will be sent to you so you can check them for the last time and answer queries that may have arisen during the layout process;
- when you send back the proofs, you will also have to send a signed copyright agreement, where you agree to transfer the copyright to the journal’s publisher;
- the PDF file will be published online. JPNIM operates under a continuous publication workflow: as soon as an article is ready to be published, it will appear in the current issue.
Screening for plagiarism
The journal staff has a policy of screening for plagiarism. All articles in this publication are original: the content (either in full or in part) in each article has not been knowingly republished without specific citation to the original release. Plagiarism checker is applied randomly to all the papers submitted.
Peer Review Process
Each unsolicited manuscript is sent to 2/3 reviewers, who evaluate it accordingly with a “single-blind peer review” procedure. For particular research papers, a review can be requested to experts in: bioinformatics, biostatistics, biophysics.
Scientific copyediting
All authors from non-English language countries should get their initial manuscript copyedited by scientific copyediting services at author's own expense. The authors have to submit the initial manuscript along with a certificate of editing. All copyediting services are fully paid for and arranged by the authors. NB. Any submission without a certificate of editing will be automatically rejected. Examples of scientific copyediting services (not affiliated nor in any way related to JPNIM): BioMed Proofreading LLC (www.biomedproofreading.com); International Science Editing Ltd. (www.internationalscienceediting.com); ScienceDocs Inc. (www.sciencedocs.com); SciTechEdit International (www.scitechedit.com); Stallard Scientific Editing (www.stallardediting.com); Eloquenti (https://eloquenti.com/editing)
Copyright and publishing rights
Regarding copyright, before publication, Authors declare that, in consideration of the action of JPNIM in reviewing and editing their submission, they transfer, assign, or otherwise convey all copyright ownership, including any and all rights incidental thereto, exclusively to the JPNIM Publisher (Hygeia Press di Corridori Marinella).
Authors have the opportunity to reuse figures, tables and selected text up to 250 words from their article as finally published, providing that full and accurate credit shall be given to publication in JPNIM and that modifications are noted (otherwise no changes may be made).
Academic re-use
Please note that authors have the opportunity to reuse figures, tables and selected text up to 250 words from their articles as finally published, providing that full and accurate credit shall be given to publication in JPNIM and that modifications are noted (otherwise no changes may be made). Translations are subject to the same rules. For all other academic re-use purposes, please send a request for permission to re-use material to journal@jpnim.com; please state “Academic re-use” in the subject line of your email.
Self-archiving
Authors can archive the published paper:
- publisher's version (the PDF published on the official journal's website) must be used;
- authors can archive it immediately (without any period of embargo);
- authors can archive it on their personal websites, institutional websites and institutional repositories;
- authors are kindly encouraged to provide a link to the journal's website.
Self-archive of preprint and postprint is not permitted instead.
JPNIM's self-archiving policy is indexed in SHERPA/RoMEO.
Archiving
Long-term preservation is ensured by Portico (a digital preservation and electronic archiving service which is part of ITHAKA, a not-for-profit organization helping the academic community use digital technologies to preserve the scholarly record and to advance research and teaching in sustainable ways).
Indexing
- JPNIM articles are indexed in Scopus (coverage begins with Volume 5, Number 1, April 2016);
- JPNIM articles are indexed in the Emerging Sources Citation Index, which is a new index within the Clarivate Analytics’ (formerly Thomson Reuters’) Web of Science (coverage begins with Volume 4, Number 1, April 2015);
- all JPNIM's articles are indexed in Google Scholar (on Google Scholar you can also see JPNIM's h5-index);
- JPNIM is listed in SJR - Scimago Journal & Country Rank;
- essential data for each JPNIM's article are indexed by mEDRA (the multilingual European Registration Agency of Digital Object Identifiers - DOIs): DOIs are assigned to all JPNIM contents;
- JPNIM is listed in the NLM (National Library of Medicine) Catalog;
- JPNIM is listed and indexed in DOAJ (Directory of Open Access Journals);
- JPNIM is listed in ROAD (Directory of Open Access Scholarly Resources);
- JPNIM is indexed in BASE (Bielefeld Academic Search Engine);
- JPNIM is listed and indexed in the Harvard Library;
- JPNIM is listed and indexed in the Yale University Library;
- JPNIM is listed in the browsable journals lists maintained by HINARI and research4life;
- JPNIM is listed in Ulrichsweb (Ulrich's periodicals database: a global serials directory):
- JPNIM is indexed in SHERPA/RoMEO (database of publishers' policies on copyright and self-archiving);
- JPNIM is listed in WorldCat;
- JPNIM is listed in the list of free medical journals about pediatrics and adolescence of The Geneva Foundation for Medical Education and Research (GFMER);
- JPNIM is listed in the Electronic Journals Library of the Simon Fraser University;
- JPNIM is listed in the Library of the University of Hong Kong;
- JPNIM is listed in the University Library of the University of Saskatchewan;
- all JPNIM's articles are indexed in CORE – Collective Goods Research & Explore (the Library of Max Planck Institute for Research on Collective Goods);
- JPNIM is listed in the GIGA Online Catalogue (of the German Institute of Global and Area Studies);
- JPNIM is listed in the WZB Electronic Journals Library (of the Social Science Research Center Berlin).
Corrections, retractions, and editorial expressions of concern
Corrections: Authors may request corrections of their work if, after publication, they find that material errors have occurred (errors that nevertheless do not question the scientific validity of the article; otherwise, retraction should be considered).
Retractions: Authors may request retraction of their work if, after publication, issues arise that call into question the scientific validity or reliability of the article. Any request for retraction by the author is subject to review and approval by the JPNIM team and the Editor-in-Chief.
Editorial expressions of concern: When concerns are noted internally or are raised by a third party, JPNIM retracts an article if the editors feel that unresolved issues in post-publication discussions warrant retraction.
Corrections and retractions are clearly noted in the journal. When an article is retracted, JPNIM publishes a retraction notice explaining the reasons for the retraction. The notice is posted at the top of the article's web page and is linked to the article's publication record. Except in rare circumstances, retracted articles remain online and available, with a clear indication of the retraction status.
Declarations to be made by the authors
Research Ethics and Publication Ethics
Authors must also comply with JPNIM’s policies on research ethics (human subjects research, animal research). Authorship is considered to be justified when the relevant Authorship and Contributorship Policy is met. In the same Policy, it is specified that one author must take primary responsibility for the entire paper, and this primary author or corresponding author must ensure that all authors meet the basic standards for authorship. It is not the responsibility of journal editors to determine who does or does not qualify for authorship. To comply with good editorial practice, the journal requires the corresponding author to sign a Publication Agreement, which confirms that all authors agree to take public responsibility for the article's content and that patient informed consent has been obtained. All authors are requested to disclose any conflicts of interest that may be inherent in the publication.
Authors are expected to be aware of and adhere to best practices in publication ethics, including, but not limited to, those related to authorship (e.g., avoiding ghost or guest authorship), dual submission, attribution, plagiarism, image integrity, and preparation of figures and competing interests: please see the Authorship and Contributorship Policy, the Author Guidelines, the Screening for plagiarism, the Conflict of Interest Statement and Funding Acknowledgement.
Authorship and Contributorship
Upon publication, the corresponding author must declare that:
- he/she is the corresponding author for this manuscript. He/she acknowledges that the corresponding author has primary responsibility for the entire paper and for correspondence with the journal, and he/she is signing this agreement on behalf of the whole group of authors;
- all authors meet the basic standards for authorship (it is not the responsibility of journal editors to determine who does or does not qualify for authorship);
- the submission has not been previously published, nor is it before another journal for consideration;
- the manuscript is an original work and I guarantee its originality. He/she guarantees that no parts have been copied by other papers, books, websites, etc.;
- he/she acknowledges that authorship credit should be based on 1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; 2) drafting the article or revising it critically for important intellectual content; and 3) final approval of the version to be published. He/she declares that each and every person listed as author meets conditions 1, 2 and 3. Moreover, he/she acknowledges that acquisition of funding, collection of data, or general supervision of the research group alone does not constitute authorship;
- all persons who qualify for authorship are listed as authors;
- he/she acknowledges that all contributors who do not meet the criteria for authorship should be listed in an Acknowledgments section. In particular, he/she acknowledges that he should declare whether assistance was received for study design, data collection, data analysis, or manuscript preparation. He/she acknowledges that, If such assistance was available, the identity of the individuals who provided this assistance and the entity that supported it in the published article should be disclosed. He/she acknowledges that financial and material support should also be acknowledged;
- he/she acknowledges that before the article final publication he/she will have to send a signed Publication Agreement, which confirms that all authors agree to take public responsibility for the article's content and that patient informed consent has been obtained; moreover, in this Publication Agreement he/she agrees on behalf of the whole group of authors to transfer the copyright to the journal’s publisher.
Moreover, he/she declares that:
- no assistance was given by people other than authors;
- OR assistance was given by people other than authors and all contributors are listed in an Acknowledgments section; he/she also certifies that these persons have given him written permission to be acknowledged.
Conflict of Interest Statement and Funding Acknowledgement
In every article a declaration of interest is published.
Upon publication, the corresponding author must declare that:
- he/she acknowledges that a conflict of interest exists when an author (or the author’s institution) has financial or personal relationships that inappropriately influence (bias) his or her actions (such relationships are also known as dual commitments, competing interests, or competing loyalties). These relationships vary from being negligible to having great potential for influencing judgment. Not all relationships represent true conflicts of interest. The potential for conflict of interest may exist regardless of whether a person believes that the relationship affects his or her scientific judgment. Financial relationships (such as employment, consultancies, stock ownership, honoraria, and paid expert testimony) are the most easily identifiable conflicts of interest and the most likely to undermine the credibility of the journal, the authors, and science itself. However, conflicts may occur for other reasons, such as personal relationships, academic competition, and intellectual passion.
The corresponding author must also certify that:
- all funding, other financial support, and material support for this work are clearly identified in the manuscript.
Moreover, the corresponding author must declare that:
- no conflicts of interest exist;
- OR conflicts of interest exist and are clearly indicated at the end of the article.
This policy is based on the "Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication - Updated April 2010" made by the International Committee of Medical Journal Editors.
Human Rights: Protection of Research Participants and Informed Consent
Considering human experimentation, the authors should write whether the procedures followed were in accordance with the ethical standards of the institutional committee on human experimentation.
If no formal ethics committee is available, the authors should indicate whether the procedures were compliant with the Helsinki Declaration as revised in 2008.
Patients have a right to privacy that should not be violated without informed consent. Identifying information (e.g., names, initials, or hospital numbers) should not be published in written descriptions, photographs, or pedigrees unless the information is needed for scientific purposes and the patient (or parent or guardian) gives written informed consent for publication.
When informed consent has been obtained, the authors should indicate it when submitting the article.
This policy is based on the "Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication - Updated December 2013" made by the International Committee of Medical Journal Editors.
Animal Rights
Considering experiments on animals, authors should indicate whether institutional and/or national standards for the care and use of laboratory animals were followed. Further guidance on animal research ethics is available from the International Association of Veterinary Editors’ Consensus Author Guidelines on Animal Ethics and Welfare.
This policy is based on the "Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication - Updated December 2013" made by the International Committee of Medical Journal Editors.
Data Availability and Sharing Policy
JPNIM encourages authors to make all data needed to replicate their study's results publicly available without restriction at the time of publication. JPNIM believes that data sharing promotes scientific progress. Authors are invited to provide a Data Availability Statement; however, publication of a Data Availability Statement is optional, as well as including a link to a data repository.